Abstract
Background
High dose chemotherapy with autologous stem cell transplant (ASCT) is a standard of care for eligible patients with multiple myeloma (MM). Adequate hematopoietic stem cell (HSC) collection is achieved by different mobilizing approaches including G-CSF, chemotherapy and plerixafor. Poor mobilizers (PM) often do not collect enough HSC after an initial attempt and need additional mobilization with or without plerixafor. However, some PM may collect enough HSC for a 2nd ASCT. The outcome of the 2nd ASCT in PM has not yet been analysed.
Methods
We retrospectively analyzed data from MM patients included in the CALM study between 2008-2012. Patients were mobilised with G-CSF +/- plerixafor, or G-CSF + chemotherapy +/- plerixafor. PM were defined as (1) patients who had a previous mobilization with insufficient peripheral blood CD34 count to proceed to apheresis, (2) patients who failed to collect sufficient HSC to be able to proceed to ASCT or (3) patients who failed to reach targeted peripheral blood CD34 count at predicted time of peak mobilization. Only PM who collected enough HSC before 1st ASCT and proceeded to a 2nd ASCT were included. Patients were not assigned randomly into group prior/after relapse for the 2nd ASCT.
Results
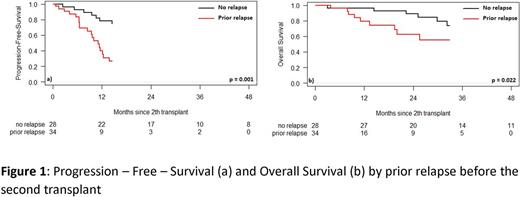
Of 499 PM, 62 patients (12.4%) had a 2nd ASCT. They collected median 7.96 (1.50-1649.00) x106/kg HSC before the first ASCT. We compared patients who relapsed before the 2nd ASCT with those who did not: the median PFS and OS were 11.1 (95% CI, 8.5-12.4) vs 29.9 (95% CI, 15.1-51.5) months (p=0.001) and 41.2 (95% CI, 9.7-undetermined) months vs median not reached (95% CI, 41.1-undetermined) (p=0.022), respectively. The median time between 1st and 2nd ASCT was 44.6 (10.8-76.1) vs 3.8 (1.6-10.8) months. Relapsing before the 2nd ASCT was important risk factor so we compared two patient groups whether they relapsed or not (55% vs 45%).
In the relapsed group (n=34), 38% of patients were mobilized with plerixafor. Patient characteristics at diagnosis were similar with regard to MM isotype, ISS stage, treatment with alkylating agents, disease stage at first collection and the number of mobilization attempts. Plerixafor group tend to collect less HSC: median 4.10 (2.02-10.90) vs 6.76 (2.02-21.82) x106/kg, p=NS. After the 1st ASCT, the plerixafor group tend to have a 2nd ASCT earlier: median interval time was 39.4 (10.8-59.5) vs 52.7 (15.5-76.1) months (p=0.111).
At 2nd ASCT, the plerixafor group patients tend to be older: 61.9 (54.5-69.1) vs 57.5 (45.8-72.5) years (p=0.22), the Karnofsky score was similar but there were more patients with advanced disease status: ≤ PR in plerixafor group 92% vs 43%, p<0.005. More patients in the plerixafor group received an alkylating agent prior to the 2nd ASCT: 50% vs 15%, p=0.097. Time to neutrophil and platelet recovery was similar. The median PFS was 11.1 months (95% IC 6.6-12.2) in plerixafor group vs 12.4 months (95% CI, 6.5-15.1) (p=0.95), and OS 41.2 (95% CI 3.1 - undetermined) months vs - not reached (95% CI, 3.8-undetermined) (p=0.23). The main cause of death in both groups was MM relapse.
In the PM group with a 2nd ASCT and without relapse after the 1st ASCT (n=28), only seven (25%) patients mobilized with plerixafor. More patients in plerixafor group receiving an alkylating agent before the 1st ASCT. Plerixafor group had more than one mobilization attempt in 17% vs 72% (p=0.05) but with less HSC collected: median 8.45 (4.97-19.84) vs 13.38 (6.15-22.30) x106/kg (p=0.04).
The age at 2nd ASCT was higher in plerixafor group: 64.5 (36.6-70.1) vs 59.3 (39.1-68.5) years (p=0.35). The neutrophil and platelet recovery was similar. For plerixafor and non plerixafor group: median PFS was 25 months (95% IC, 7.6- 51.6) vs 31 months (95% IC, 22.3-51.5) (p=0.78) and OS at 12 months after the 2nd ASCT 100% (95% IC, 0.0 - 100.0) vs 95.2 (95% IC, 86.1-100.0), respectively.
Conclusions
A second ASCT in poor mobilizers myeloma patients is feasible, though rarely done. Relapsing before 2nd ASCT is the most important risk factor for a shorter PFS and OS. PM mobilized with plerixafor regardless of relapse prior to 2nd ASCT had a trend to be older at transplant, with higher disease stage at diagnosis and are more often treated with an alkylating agent. They needed less mobilization attempts and collected less HSC, however, still sufficient for two ASCTs. There were no differences between PFS and OS within the relapsed and non-relapsed PM groups with regard whether or not they received plerixafor.
Sever: Celgene: Honoraria; Amgen: Honoraria; Novartis: Honoraria. Apperley: Sun Pharma: Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Incyte: Honoraria; Therakos: Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Other: travel, accommodations, expenses , Research Funding, Speakers Bureau; Ariad: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Mohty: Sanofi: Honoraria, Speakers Bureau. Scheid: Takeda: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Kobbe: Celgene: Other: Advisory Board, Research Funding. Mayer: Eisai: Research Funding; Novartis: Research Funding. Trneny: Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Schönland: prothena: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Speakers Bureau; Sanofi: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Garderet: Takeda: Honoraria; Amgen: Honoraria. Kroeger: Neovii: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal